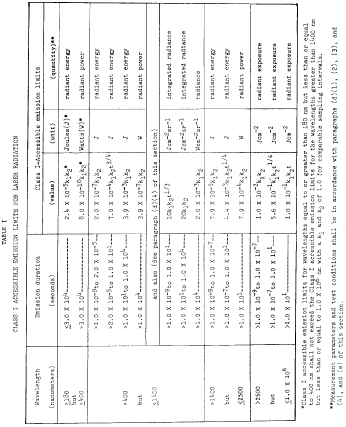
GMP Regulation Handbook: Electronic Signatures, 21 CFR Part 11 | ISPE | International Society for Pharmaceutical Engineering
FDA 21 CFR Part 11 Training, Regulations, and Best Practices - GxP Training : Certified Online Courses for Life Sciences